

Protective measures in the medical device itself or in the manufacturing process
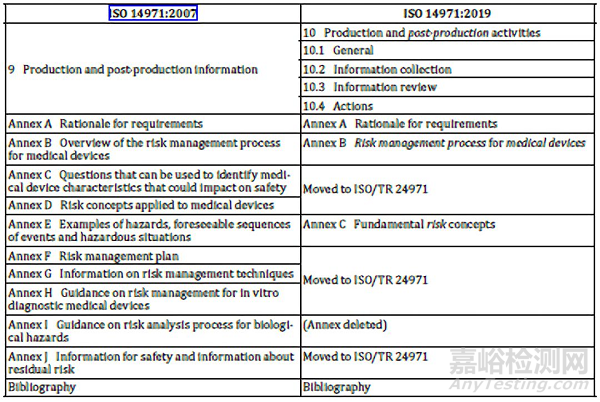

In 2012, a European harmonized version of this standard was adopted by CEN as EN ISO 14971:2012. Specifically, ISO 14971 is a nine-part standard which first establishes a framework for risk analysis, evaluation, control, and review, and also specifies a procedure for review and monitoring during production and post-production. Such activity is required by higher level regulation and other quality management system standards such as ISO 13485. This standard establishes the requirements for risk management to determine the safety of a medical device by the manufacturer during the product life cycle. In 2013, a technical report ISO/TR 24971 was published by ISO TC 210 to provide expert guidance on the application of this standard. The latest significant revision was published in 2019. This standard is the culmination of the work starting in ISO/IEC Guide 51, and ISO/IEC Guide 63. The ISO Technical Committee responsible for the maintenance of this standard is ISO TC 210 working with IEC/SC62A through Joint Working Group one (JWG1). ISO 14971 Medical devices - Application of risk management to medical devices is an ISO standard for the application of risk management to medical devices. What are you waiting for? All the ISO 14971 2016 PDF free download and audio books you need, now at your fingertips on stuvera site! ABOUT THE BOOK ISO 14971 2016 PDF free download I bring you the latest information on this Ebook site called Stuvera where you can download ISO 14971 2016 PDF free download without any cost or registration. Do you happen to have been searching for a website where you can download ISO 14971 2016 PDF free download in PDF paying a cent?.


 0 kommentar(er)
0 kommentar(er)
